Abstract
Introduction Detection of molecular relapses may allow early intervention aiming improved survival or , at least, reduced toxicities. However, so far the impact of molecular relapses in pediatric AML is not yet determined. Therefor this retrospective analysis outlines the prediction of clinical relapses following molecular relapses in pediatric patients inv(16), t(8;21), t(9;11), t(10;11) or NPM1.
Patients and Methods Patients included in this analysis was initially diagnosed with an AML according to WHO with at least one quantitative marker, availability of an initial sample (day 0) and more than three follow up measurements until 18 months (d=532) after date of diagnosis. Between 2015 and 2020 n=258 children with inv(16) (n=52; 20%), t(8;21) (n=84; 32%), t(9;11) (n=64;25%), t(10;11) (n=20; 8%) and NPM1 (n=38; 15%) underwent MRD monitoring by rt-PCR in bone marrow (BM). Monitoring in peripheral blood (PB) was also recommended every 4 weeks. In all patients (molecular) CR must be confirmed at the start of last consolidation. Based on the marker specific sensitivity a molecular relapse was defined as an increase in MRD level by at least 1 log to a level greater than the marker specific baseline, with a normal percentage (<5%) of myeloblasts in BM and the absence of proven histological extramedullary relapse. Patients were censored if they had a clinical relapse or reach the age of 18 years.
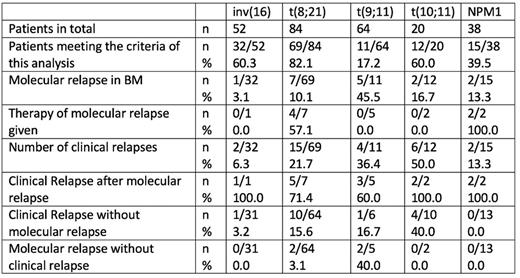
Results For inv(16) 60% (n=32), t(8;21) 82% (n=69), t(9;11) 17% (n=17), t(10;11) 60% (n=12) and NPM1 39% (n=15) of the patients meet the inclusion criteria.
In inv(16) patients, n=1 (3%) molecular relapse could be detected, which was followed within 4 weeks by a clinical relapse. One clinical relapse was recorded 146 days after an increasing signal in PB, but without a corresponding molecular relapse. In the t(8;21) cohort, n=7 patients was diagnosed with a molecular relapse, 4/7 were treated pre-emptive with azacytidine. 5 patients got a clinical relapse afterwards, n=2 did not relapse open until the end of the observational timeframe to d532. In n=10 patients the clinical relapse could not be predicted by rt-PCR. For MLL-rearranged AML such as t(9;11) and t(10;11) molecular relapses was diagnosed in n=5 (45%) and n=2 (17%) patients. None of them was pre-emptively treated. In 60% (n=3) for t(9;11) and 100% (n=2) for t(10;11) clinical relapses followed. Interestingly, that just one patient (17%) with t(9;11) had a clinical relapse without a prior molecular relapse, but in 40% (n=10) of the t(10;11) group, the clinical relapse was not molecularly predictable. In n=2 of the molecular relapses in t(9;11) patients no clinical relapse followed within the observation period. In NPM1 positive patients n=2 molecular relapses (13%) were diagnosed, both were treated with azacitidine, both relapsed open. In this group was no patient with a clinical relapse without a prior molecular relapse.
Conclusion In all groups MRD level measured by rt-PCR could be an effective diagnostical tool in pediatric AML to detect molecular relapses prior to clinical relapses opening a window for pre-emptive therapy. Obviously in MLL-rearranged AML like t(9;11) or t(10;11) kinetics seems to be too fast (at least for an epigenetic intervention) that, once the molecular relapse is detected, clinical relapse follows immediately or, the MRD levels increase so rapidly, that the molecular relapse cannot be detected within the recommended analysis interval of 4 weeks. Same seems to be apply to patients with NPM1. In core-binding leukemia such as inv(16) and t(8;21) the kinetics leads to enough time to step into the pre-emptive therapy with azacitidine. Here is predictability pending on a close monitoring in PB and BM, which needs to be implemented without time gaps in sampling in everyday clinical practice to keep this additional treatment chance for patients. Even if exemptions are very rare (3%) and can be most likely explained by errors during lab measurement, molecular relapses in pediatric patients with inv(16), t(8;21), t(9;11), t(10;11) or NPM1 leads to clinical relapses over time.
Disclosures
Reinhardt:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cerus: Membership on an entity's Board of Directors or advisory committees; Medac: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding.
OffLabel Disclosure:
Azacitidine to treat pediatric AML molecular relapse.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal